*TLA = Three-Letter Acronym
Special thanks to Sandra Spivey for spurring me to expand upon this important topic at long last!
I’m very interested in your ideas on dose finding methods. For too long we’ve relied on vague “maximum tolerated dose” voodoo. Tolerated in whose eyes? Maximum - why? We don’t use maximum tolerated dose for OTCs like Tylenol. Why for cancer drugs? #bcsm
— Sandra Spivey (@SpiveySandra) July 22, 2019
Sandra’s multifaceted question demands a clear exposition of what I have termed the MTD heuristic. As Sandra indicates, the MTD concept has acquired something of a murky and esoteric character. I’ll try to pierce the fog in 3 steps: heuristics, history and histrionics.
Heuristics
The word heuristic is cognate to Archimedes’ “Eureka!” (“I have found it!”) which he famously shouted as he ran naked through the streets of Syracuse, upon discovering the principle of buoyancy in his bath. My own usage of the word follows George Pólya’s treatment in his classic book How to Solve It, where he describes heuristics as general patterns of behavior you can adopt consciously when solving problems. In the book, Pólya focuses on math problems, but the notion applies in all kinds of problem-solving activity.
For example, when Sir William Osler (the ‘Father of Modern Medicine’) said “Listen to your patient; he is telling you the diagnosis”, he was articulating a diagnostic heuristic that focuses the physician’s attention on the words and concerns as expressed by the patient.
In the question of dosing chemotherapy drugs, the maximum-tolerated-dose heuristic embodies the view that chemotherapy is a crude instrument—a cytotoxic agent that hurts all the cells of the body, but hurts cancer cells most of all. If a cancer is deadly, and the intent of treatment is to cure—i.e, to eradicate the cancer—then it seems reasonable to give this poison in a series of massive, barely-tolerable doses seprarated by brief intervals when the patient (but not the cancer, we hope) can recover. These are of course the familiar ‘cycles’ of chemo.
It should be said that general aspects of this heuristic apply outside of cancer treatment. Hippocrates himself said, “For extreme diseases, extreme methods of cure, as to restriction, are most suitable.” I’m not quite sure what he meant by “as to restriction,” but certainly this calls to mind the dreadful story of how—before the discovery of insulin—children with diabetes were treated by starvation.
The MTD heuristic also generalizes even beyond medicine. Chemo cycles are a medical example of a widely applicable engineering principle called a bang-bang control. Under some models of cancer growth and treatment, one can prove mathematically that such chemo regimens are optimal.
But of course, when you do math, the results you get out exactly match the assumptions you put in. When you change your assumptions about cancer growth and treatment, the math may show that other heuristics work best. One much-discussed alternative is metronomic chemotherapy, almost the exact opposite of traditional chemo cycles driven by the MTD heuristic:
Metronomic chemotherapy is based on the chronic administration of chemotherapeutic agents at relatively low, minimally toxic doses, and with no prolonged drug-free breaks
Pasquier E, Kavallaris M, André N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol. 2010 Aug;7(8):455–65. PMID 20531380
I mention metronomic chemo here only because helps me to put the MTD heuristic into context as merely one of several heuristics that may apply in cancer treatment. Most of my further discussion here will assume that the basic assumptions upholding the MTD heuristic are correct. Chief among these is that treatment is being pursued with curative intent.
History
Emil Frei’s 1985 paper “Curative Cancer Chemotherapy” explains the rationale for high-dose combination chemotherapy regimens, as emerging from the achievement of “curative treatment for some 12 categories of cancer.” In this review, Frei appeals repeatedly to “steep” dose-response curves, often juxtaposing toxicity with efficacy by mentioning patient and tumor responses in the same breath:
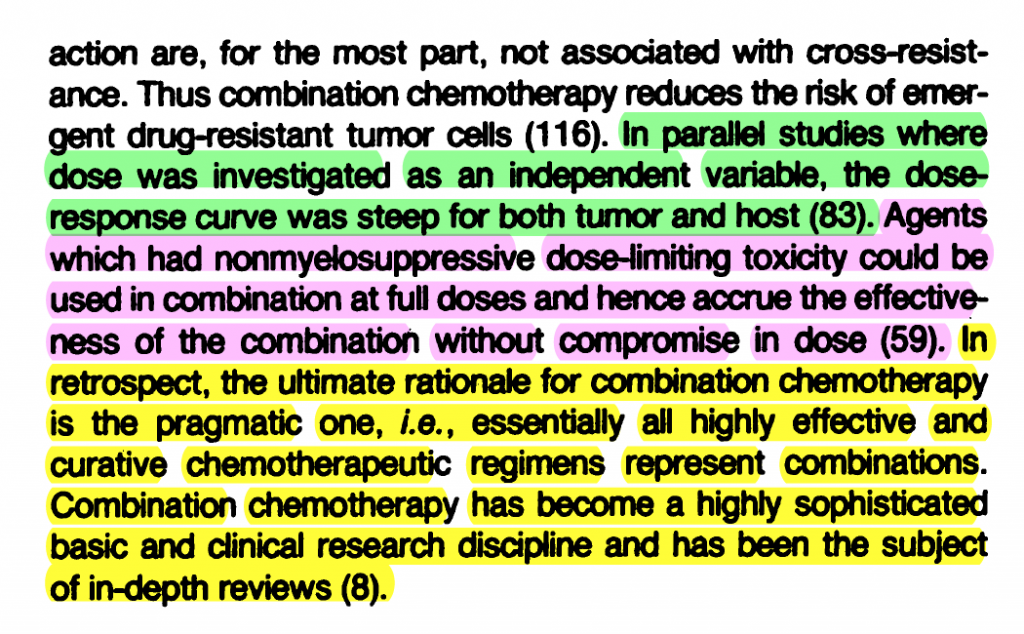
1.
Frei E. Curative cancer chemotherapy. Cancer Res. 1985 Dec;45(12 Pt 1):6523–37.
Frei’s paper is open-access, and it seems to me eminently readable for a cancer patient advocate. I’ve also posted my own PDF copy in case my highlights and notes would be of any use in focusing on questions related to dose-response.
Histrionics
The Winter 2018–19 issue of CancerWorld newsletter featured a cover story ostensibly challenging the MTD heuristic via a “minimum effective dose” (MED) concept:
… okay, “non-story” now seems a bit harsh as I read 2nd half. Once you get past the TLA-juggling conceit (‘the’ MTD vs ‘the’ MED), the piece moves into substantive territory. The interview with @HansScheurer of @MyelomaEurope offers crucial insights: pic.twitter.com/zWcmSryPsk
— David C. Norris, MD moved to Mastodon 🦣 (@davidcnorrismd) December 4, 2018
As noted in my hot-take tweeting above, the article did eventually move into substantive territory. But here I want to probe its problematic framing in terms of the murky notion of minimum effective dose.
The words “minimum effective dose” sound so appealing. A minimal dose that’s also effective! What more could one ask? But if you probe this phrase with any amount of mathematical discipline, it becomes basically meaningless. Here are 3 possible meanings that “minimum effective dose” could conceivably have:
- MED1: The smallest dose that delivers the drug’s full cancer-killing effect
- MED2: The smallest dose that has a detectable effect
- MED3: The smallest dose that achieves a ‘reasonable’ effect
So long as the dose-response curve eventually reaches a plateau, an MED1-type dose will exist. But the plateau might happen at a very high dose that isn’t tolerable, or even survivable. So much for the reassuring word “minimum”! On the other hand, an MED2-type dose that produces a just-barely-detectable effect might not be worth the trouble of going to the infusion center, etc. So much for “effective”!
Of these, the only one that has a chance of making any sense at all is MED3. But obviously there is a lot of wiggle room in ‘reasonable’. Maybe reasonable means the effect that some wonky economists think is good enough for the general public? So how about calling it MED3e, where the ‘e’ stands for economist?
Okay then, how about if we let it mean the effect each patient thinks is reasonable? That certainly starts to sound promising, and indeed I think it is. But on this interpretation, the MED3 concept starts to look an awful lot like an individualized MTD, or MTDᵢ — where each patient decides the question of ‘tolerability’. Let’s call this interpretation MED3ᵢ.
This is because, as a rule, both toxicity to the patient and ‘toxicity to the cancer’ (i.e., efficacy) will increase with bigger doses of an anti-cancer drug. So the patient deciding what is ‘reasonable’ or ‘tolerable’ will have to balance toxicity against hoped-for efficacy. This trade-off remains essential and salient, regardless of how the patient’s dosing heuristic is worded:
- “I will choose the lowest dose that will give me the level of efficacy I am hoping for” (MED3ᵢ)
- “I will choose the highest dose that feels tolerable to me in light of the efficacy I am hoping for” (MTDᵢ)
To the extent that these two formulations differ, the difference favors MTDᵢ. This is because the toxicities are likely to be more definite and experienced in the near term, whereas future efficacy is indefinite and unpredictable. It seems to me that focusing individualized dosing decisions on value-judgements about toxicity experienced in the present will produce clearer thinking than keying these decisions on a fixed level of hoped-for future efficacy.
All this brings us back to yet another perspective on heuristics. We humans can’t (and don’t) make hard decisions by completely rational calculations.
Captain James T. Kirk : What would you say the odds are on our getting out of here?
Mr. Spock : Difficult to be precise, Captain. I should say, approximately 7,824.7 to 1.
Like the situations Captain Kirk always seemed to be getting into, dosing of cancer treatments is a deep problem that you can’t just ‘calculate your way out of’. Ideally, dosing these drugs involves integrating an individual patient’s value judgments together with profoundly uncertain clinical predictions. Humans solving such problems use rough rules of thumb. To be sure, MED3ᵢ and MTDᵢ are both rough heuristics. Furthermore, they both represent ways to deal with the same fundamental trade-off between toxicity and efficacy. But my argument above shows that MED3ᵢ rests on an ambigous phrase “minimum effective dose” with words chosen for emotional appeal (‘histrionics’) instead of conceptual clarity.
This post is still a work-in-progress, but I’ll post it now to get some feedback on how to focus it better, or where to elaborate. There’s so much more to say here, including connections I would like to draw between some of the worst thinking in regard to cancer drug dose-response and homeopathy.
